Overview
HealthiView includes a built-in REST API to allow for programmatic access to your data using any language or platform
which supports the secure HTTP protocol and can process JavaScript Object Notation (JSON).
Use the API to retrieve any of the following:
- Your current study list
- Details for any study
- The list of surveys for a study
- The configuration of a survey
- Detailed response data for a single survey, or across multiple surveys
- Study, survey and site-level metrics
API requests are made against our live production database, which is updated nightly with the most recent response data
for all active surveys.
See
Request Limits below for more information about API availability
and request restrictions.
Authentication
The REST API is a stateless interface, and requires that you provide an authentication signature
with every request. This signature is made up of your unique API Key, along with a
generated verifier string based on your unique API Secret.
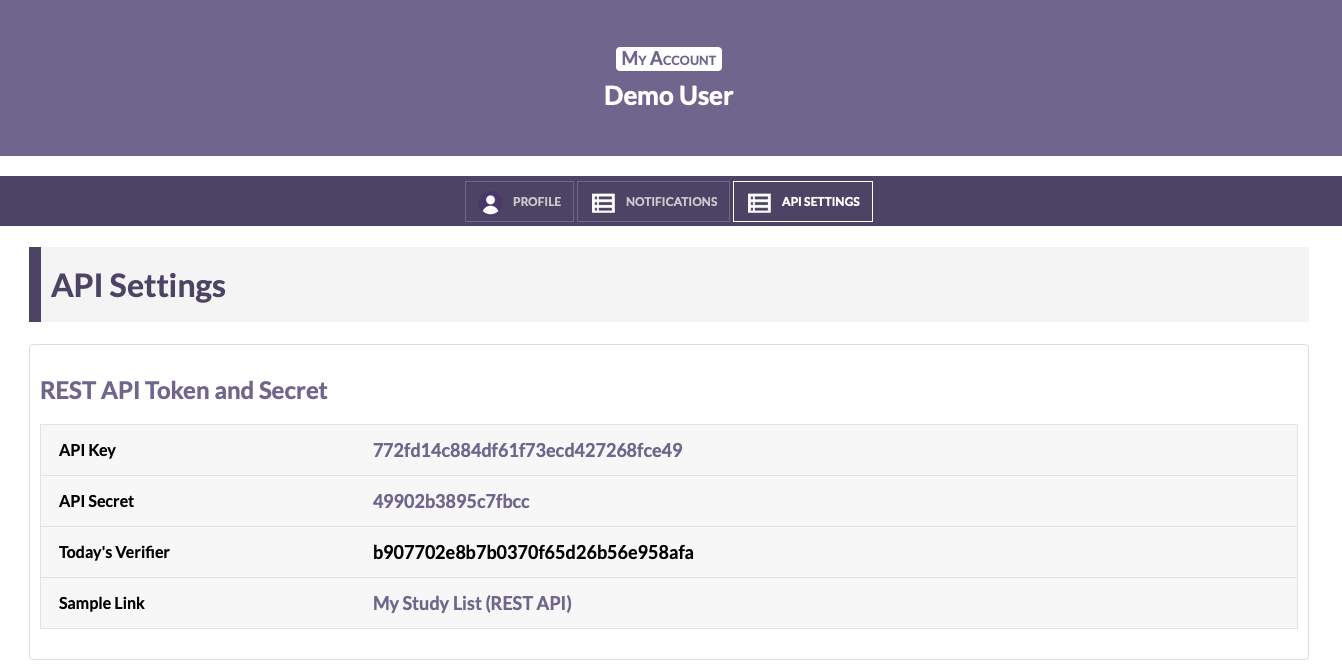
Find your API Key and API Secret on your profile page:
If you don't see your API Key and Secret on your profile page,
your HealthiView user account may not include API access. Contact your CorEvitas Project Manager
for more information.
The verifier string is a MD5 hash based on the API Secret in combination
with the current UTC date, in YYYYMMDD format. Including the date helps secure your data by preventing
old verifier strings from ever being re-used.
The following example, in the PHP programming language, illustrates how to construct a verifier string:
$apiSecret = "MY_API_SECRET";
$verifier = md5($apiSecret . gmdate("Ymd"));
These two components, the API Key and the Verifier, are appended to each Request as query string parameters:
/REST_API_PATH/?api_token={$ApiKey}&verifier={$verifier}
Click the API Secret in your profile page to see the current day's verifier,
generated using the algorithm above.
Request Syntax
API methods return study, survey, site and response-level data.
Each method returns either a single JSON object, or an array of objects.
- For the Study object, either an array of all studies or a single Study detail can be returned
- For the Survey object, an array of surveys for a single study can be returned, or a single survey detail
- For the Response object, an array of responses may be returned for a single survey, or across all studies
Each object type has a corresponding REST signifier (study, site, survey, response). To request a particular object,
append the REST components together in hierarchical form, separating each object signifier with the unique identifier for that object.
Note that in the examples that follow, the request signature is omitted. See Authentication for full details.
/REST_API_PATH/study/studyIdentifier/survey/surveyIdentifier
/REST_API_PATH/study/studyIdentifier/survey/surveyIdentifier/response
When requesting lists, paging will be used to return the data. Therefore, it's important to check the total_pages attribute of the return object header.
To request additional pages, add a page query string attribute to the request:
/study/studyIdentifier/survey?page=2
If the requested page is not valid, the last valid page will be returned, and this will be identified in the page attribute of the return object. See the Return Format section for details.
See the Methods section for details on each request method.
Verbose Option
When available, a verbose parameter can be added to a request, set to true, to increase the detail of the repsponse.
/study?verbose=true
See the individual methods for the additional data available using the verbose option.
Return Format
All data requests are returned as JSON-encoded strings. The following fields are included in the result object.
Note that the return object includes a nonce, which is a randomly generated one-time use string; and a verifier, which is
a HMAC-style SHA256 verification string based on the nonce and the API secret.
| Field |
Type |
Description |
| result_ok |
Boolean |
true if the call (including authentication) succeeded; otherwise false |
| utc |
Integer |
UTC seconds since epoch (January 1, 1970) |
| reason |
String |
Text description of the error if result_ok is set to false; otherwise omitted |
| request |
String |
One of: study, site, survey, response, score - the data element requested |
| return_type |
String |
array or object; the JSON type of the data member of the return object, based on the request |
| api_token |
String |
The public API key associated with the request |
| nonce |
String |
Random alphanumeric sequence used, in conjunction with the requestor's private API secret, to generate the verifier as a HMAC-style hash |
| verifier |
String |
HMAC-style keyed hash value, using SHA256 algorithm, and generated using the nonce and the API secret |
| page |
Integer |
Page number of the returned data; will always be 1 when return_type is object, otherwise will be the requested page if available |
| total_pages |
Integer |
Total number of available pages to be returned |
| api_requests_24hour |
Integer |
Total number of successful API requests made in the past 24 hours |
| api_requests_30day |
Integer |
Total number of successful API requests made in the past 30 days |
| response_time_ms |
Integer |
Elapsed time, in milliseconds, for the server to process the request |
| data |
Mixed |
A JSON Array or a JSON object, depending on the request, containing the requested list or detail object |
The following is an example return object in JSON format:
{
"result_ok": true,
"utc": 1523041919,
"api_client": "CorEvitas",
"request": "study",
"return_type": "object",
"page": 1,
"total_pages": 1,
"api_requests_24hour": 15,
"api_requests_30day": 120,
"response_time_ms": 125,
"api_token": "772fd14c884df61f73ecd427268fce49",
"nonce": "20c2ceed400cf2b4",
"verifier": "5a7aa520d532bafca9b21a6c6c5b1a171b1277e2ebe9575afa1d45ceff72f3c4",
"data": {
}
}
Limits
Daily and monthly API request limits are in place to ensure data availability for all our clients,
and for HealthiView application users.
Any client with API access can make, without additional fees, up to 500 successful REST API calls in
any 24-hour window, provided no more than 15000 total REST API calls are made in the current 30-day window.
Use the api_requests_24hour and api_requests_30day properties of the return object
to keep track of the number of API requests.
Methods
Get Study List
| Syntax | /study?verbose=true |
| Returns | Array |
| Description | Returns an array of Study object identifiers. The array will include all studies
associated with the client account, active or closed. |
Study Array Object Format
| Field |
Type |
Description |
| key |
String |
Internal study identifier, auto-generated |
| name |
String |
Descriptive study name |
| status |
String |
Active or Closed |
| surveys |
Array |
Available when verbose option is used - List of surveys associated with the study. Each Survey object includes a unique key
which can be used for additional API requests, along with a descriptive name and survey status. |
Payload Example
{
"result_ok": true,
"utc": 1526386895,
"api_client": "CorEvitas",
"request": "study",
"return_type": "array",
"page": 1,
"total_pages": 1,
"total_items": 4,
"api_token": "772fd14c884df61f73ecd427268fce49",
"nonce": "c608810056f022f4",
"verifier": "4d8526e50faa682d8aecd7c161153d0f174ad139c79257aff1bdddb11fbcbc6f",
"data": [{
"key": "201710-59da721e7b2b8",
"name": "Polaris Demo",
"status": "Active"
}, {
"key": "HV-EXIT-DEMO",
"name": "T10001 Sample Study 1",
"status": "Active"
}, {
"key": "HV-TEST-LOW",
"name": "T10002 Sample Study 2",
"status": "Active"
}, {
"key": "HV-DEMO-1",
"name": "T10003 Sample Study 3",
"status": "Closed"
}]
}
Get Study Detail
| Syntax | /study/identifier |
| Returns | Object |
| Description | Returns a single Study object, including an array of Survey objects. |
Study Detail Object Format
| Field |
Type |
Description |
| key |
String |
Internal study identifier, auto-generated |
| name |
String |
Descriptive study name |
| status |
String |
Active or Closed |
| openDate |
String |
Date the survey was launched (YYYY-MM-DD HH:MM:SS) |
| closeDate |
String |
Date the survey was closed, if status is Closed; otherwise null |
| firstResponse |
String |
Date the earliest survey response was submitted (YYYY-MM-DD HH:MM:SS) |
| lastResponse |
String |
Date the most recent survey response was submitted (YYYY-MM-DD HH:MM:SS) |
| numResponses |
Number |
Total number of responses received, to date |
| metrics |
Object |
Latest metrics captured for the study, including average response time (minutes), mean score,
standard deviation of the mean score, and margin of error. May be null if no responses
have been received. |
| surveys |
Array |
List of surveys associated with the study. Each Survey object includes a unique key
which can be used for additional API requests, along with a descriptive name |
Payload Example
{
"result_ok": true,
"utc": 1526386040,
"api_client": "CorEvitas",
"request": "study",
"return_type": "object",
"page": 1,
"total_pages": 1,
"total_items": 2,
"api_token": "772fd14c884df61f73ecd427268fce49",
"nonce": "1fa41dc7b106b5d5",
"verifier": "b1e855a38c502e1afc685a8b68c5615ed03aff0f9a73eb20d12b1939795891a3",
"data": {
"key": "201710-59da721e7b2b8",
"name": "Polaris Demo",
"status": "Active",
"openDate": "2017-09-01 00:00:00",
"closeDate": null,
"firstResponse": "2017-10-08 17:45:21",
"lastResponse": "2017-10-08 18:08:12",
"numResponses": 12,
"metrics": {
"avgResponseTime": 2.0333333333333337,
"mean": 3.5143,
"stddev": 1.031691932378853,
"margin": 0.1438820777250121
},
"surveys": [{
"key": "201710-451b482ff619c",
"name": "Intro Survey"
}, {
"key": "201710-59da722fe647e",
"name": "Exit Survey"
}]
}
}
Get Survey Detail
| Syntax | /study/identifier/survey/identifier |
| Returns | Object |
| Description | Returns a single Survey object. Includes survey metadata,
latest reported metrics, and the complete survey configuration with all
questions and available response options. |
Survey Detail Object Format
| Field |
Type |
Description |
| key |
String |
Internal survey identifier, auto-generated |
| name |
String |
Descriptive survey name, unique within the study, typically related to the survey type |
| active |
Boolean |
true if the survey is currently open, otherwise false |
| hidden |
Boolean |
true if the survey is hidden from logged-in users (typically during intialiazation), otherwise false |
| indexOrder |
Number |
The sequence number of the survey, used for display purposes, where a higher number indicates a survey fielded later in the study |
| surveyType |
String |
One of: Intro, Midpoint, Exit or Custom |
| numResponses |
Number |
Total number of responses received, to date |
| creationdate |
Date |
Date the survey was added to the system (YYYY-MM-DD HH:MM:SS) |
| metrics |
Object |
Latest metrics captured for the survey, including average response time (minutes), mean score,
standard deviation of the mean score, and margin of error. May be null if no responses
have been received. |
| config |
Object |
See the Survey Config Detail Object Format below |
Survey Config Detail Object Format
| Field |
Type |
Description |
| name |
String |
Descriptive survey config name, typically related to the survey type |
| surveyType |
String |
One of: Intro, Midpoint, Exit or Custom |
| questions |
Array |
List of all questions, including metadata, presented in sequential order |
question
key |
String |
Unique question key within the system. Typically composed of a standard (HV) or client-based prefix code,
followed by a theme abbreviation and an auto-incremented numeric suffix. |
question
question |
String |
Exact text of the question as presented to respondents |
question
theme |
String |
The theme associated with the question for the purpose of aggregating metrics |
question
scored |
Boolean |
true if the question is used in calculation of metrics, typically
for Likert-scale based satisfaction questions, otherwise false |
question
options |
Array |
Fixed response options for the question, presented as an array of strings; may be omitted
if no fixed response options are applicable for the question |
Payload Example
{
"result_ok": true,
"utc": 1526387294,
"api_client": "CorEvitas",
"request": "survey",
"return_type": "object",
"page": 1,
"total_pages": 1,
"total_items": 1,
"api_token": "772fd14c884df61f73ecd427268fce49",
"nonce": "fb116db624d17d11",
"verifier": "882a5c13df29df2a81032be39a6ab328d705e0b90018e648800ef75069de058b",
"data": {
"key": "201710-59da722fe647e",
"name": "Exit Survey",
"active": true,
"hidden": false,
"indexOrder": "1",
"surveyType": "Exit",
"creationdate": "2017-10-08 14:45:34",
"metrics": {
"avgResponseTime": 2.0333333333333337,
"mean": 3.5143,
"stddev": 1.031691932378853,
"margin": 0.1438820777250121
},
"config": {
"name": "Exit Survey",
"surveyType": "Exit",
"questions": [{
"key": "HV-META1",
"question": "Response ID",
"theme": "Meta Data",
"scored": false
}, {
"key": "HV-META2",
"question": "Start Time",
"theme": "Meta Data",
"scored": false
}, {
"key": "HV-META3",
"question": "Finish Time",
"theme": "Meta Data",
"scored": false
}, {
"key": "HV-META4",
"question": "Status",
"theme": "Meta Data",
"scored": false,
"options": ["Complete", "Partial", "Disqualified"]
}, {
"key": "HV-META6",
"question": "Language",
"theme": "Meta Data",
"scored": false
}, {
"key": "HV-DEM1",
"question": "What country are you in?",
"theme": "Demographic",
"scored": false
}, {
"key": "HV-SCR1",
"question": "Would you like to participate in the survey?",
"theme": "Profile Questions",
"scored": false,
"options": ["Yes", "No"]
}, {
"key": "HV-META10",
"question": "Please select your study site from the list below.",
"theme": "Meta Data",
"scored": false
}, {
"key": "HV-DEM2",
"question": "What is your gender?",
"theme": "Demographic",
"scored": false,
"options": ["Male", "Female"]
}, {
"key": "HV-DEM9",
"question": "How old are you?",
"theme": "Demographic",
"scored": false,
"options": ["Under 18", "18-21", "22-29", "30-39", "40-49", "50-59", "60-69", "70-79", "80-89", "90-99", "100+"]
}, {
"key": "HV-DEM7",
"question": "What motivated you to join the clinical trial? (Select all that apply)",
"theme": "Demographic",
"scored": false,
"options": ["Curiosity to see if it would improve my condition", "Contribute to research and help people live longer", "To seek a better quality of life", "No other treatment options were available", "Free medication and\/or treatment", "Free procedures", "Compensation", "Other", "None of the above"]
}, {
"key": "HV-COM1",
"question": "During the informed consent process, how satisfied were you with the explanation of the study?",
"theme": "Communication",
"scored": true,
"options": ["Not at all satisfied", "Slightly satisfied", "Moderately satisfied", "Very satisfied", "Completely satisfied"]
}, {
"key": "HV-SE18",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Good lighting in waiting area and exam rooms",
"theme": "Site Experience",
"scored": true,
"options": ["Not at all satisfied", "Slightly satisfied", "Moderately satisfied", "Very satisfied", "Completely satisfied", "Not Applicable"]
}, {
"key": "HV-SE19",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Close and convenient access to restrooms",
"theme": "Site Experience",
"scored": true,
"options": ["Not at all satisfied", "Slightly satisfied", "Moderately satisfied", "Very satisfied", "Completely satisfied", "Not Applicable"]
}, {
"key": "HV-SE20",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Amenities such as WIFI, magazines, TV",
"theme": "Site Experience",
"scored": true,
"options": ["Not at all satisfied", "Slightly satisfied", "Moderately satisfied", "Very satisfied", "Completely satisfied", "Not Applicable"]
}, {
"key": "HV-SE21",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Cleanliness of facilities",
"theme": "Site Experience",
"scored": true,
"options": ["Not at all satisfied", "Slightly satisfied", "Moderately satisfied", "Very satisfied", "Completely satisfied", "Not Applicable"]
}, {
"key": "HV-SE22",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Site accommodations (comfortable seating, private space, etc.)",
"theme": "Site Experience",
"scored": true,
"options": ["Not at all satisfied", "Slightly satisfied", "Moderately satisfied", "Very satisfied", "Completely satisfied", "Not Applicable"]
}, {
"key": "HV-SE23",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Access to drinks and snacks",
"theme": "Site Experience",
"scored": true,
"options": ["Not at all satisfied", "Slightly satisfied", "Moderately satisfied", "Very satisfied", "Completely satisfied", "Not Applicable"]
}, {
"key": "HV-OS1",
"question": "How satisfied were you with your overall clinical research study experience?",
"theme": "Overall Satisfaction",
"scored": true,
"options": ["Not at all satisfied", "Slightly satisfied", "Moderately satisfied", "Very satisfied", "Completely satisfied"]
}, {
"key": "HV-REL1",
"question": "How satisfied were you with your health concerns being addressed by the study staff?",
"theme": "Relationship building and support",
"scored": true,
"options": ["Not at all satisfied", "Slightly satisfied", "Moderately satisfied", "Very satisfied", "Completely satisfied"]
}, {
"key": "HV-SE7",
"question": "How often was the study staff prepared for your visits?",
"theme": "Site Experience",
"scored": true,
"options": ["Never", "Rarely", "Sometimes", "Often", "Always"]
}, {
"key": "HV-COM4",
"question": "How satisfied were you in general with how study staff communicated with you?",
"theme": "Communication",
"scored": true,
"options": ["Not at all satisfied", "Slightly satisfied", "Moderately satisfied", "Very satisfied", "Completely satisfied"]
}, {
"key": "HV-CON10",
"question": "How easy was it taking your study medication (e.g. swallowing, injecting, etc.)?",
"theme": "Convenience",
"scored": true,
"options": ["Not at all easy", "Slightly easy", "Moderately easy", "Very easy", "Extremely easy"]
}, {
"key": "HV-COM5",
"question": "How satisfied were you with the instruction provided by the study staff for how to take and store your study medication?",
"theme": "Communication",
"scored": true,
"options": ["Not at all satisfied", "Slightly satisfied", "Moderately satisfied", "Very satisfied", "Completely satisfied"]
}, {
"key": "HV-CON5",
"question": "How convenient was parking at the study site?",
"theme": "Convenience",
"scored": true,
"options": ["Not at all convenient", "Slightly convenient", "Moderately convenient", "Very convenient", "Extremely convenient", "Not applicable"]
}, {
"key": "HV-REL8",
"question": "How helpful would the following be to support your participation in the clinical trial? Please rank the following items in order from most to least supportive.",
"theme": "Relationship building and support",
"scored": false,
"options": ["Travel Coordination", "Vouchers\/pre-payment", "Home visits or alternate location visits", "24\/7 telephone support"]
}, {
"key": "HV-REL3",
"question": "How often did you feel encouragement and support from the study staff?",
"theme": "Relationship building and support",
"scored": true,
"options": ["Never", "Rarely", "Sometimes", "Often", "Always"]
}, {
"key": "HV-CON9",
"question": "How easy was it to arrange child care or spouse\/parent care while you went to your study visits?",
"theme": "Convenience",
"scored": true,
"options": ["Not at all easy", "Slightly easy", "Moderately easy", "Very easy", "Extremely easy", "Not applicable"]
}, {
"key": "HV-SE11",
"question": "Were you encouraged to bring a friend or family member to the study visits?",
"theme": "Site Experience",
"scored": true,
"options": ["Not at all encouraged", "Slightly encouraged", "Moderately encouraged", "Very encouraged", "Completely encouraged"]
}, {
"key": "HV-HLP1",
"question": "Did you ever consider stopping your participation in the clinical trial?",
"theme": "Helping self and others",
"scored": false,
"options": ["Yes", "No"]
}, {
"key": "HV-HLP6",
"question": "Which of the following, if any, were reasons why you considered stopping your participation in the clinical trial? (Select all that apply)",
"theme": "Helping self and others",
"scored": false,
"options": ["Family or caregiver asked me to stop", "Too many study visits were required", "I thought that I was on a placebo (sugar pill, inactive pill)", "Missed too much work", "Transportation or travel distance was a burden", "Lack of extended office hours", "I did not have child care", "The study staff were not friendly", "Too much time involved", "I did not feel appreciated", "The study site was not convenient for me", "Instructions were too difficult to follow", "Worried about side effects", "I felt I was not being informed about my health", "Frequency or complexity of informed consent\/re-consent updates", "I don't remember", "Other"]
}, {
"key": "HV-HLP3",
"question": "How likely are you to participate in another clinical trial?",
"theme": "Helping self and others",
"scored": true,
"options": ["Not at all likely", "Slightly likely", "Moderately likely", "Very likely", "Extremely likely"]
}, {
"key": "HV-HLP4",
"question": "Why would you participate in another clinical trial? (Select all that apply)",
"theme": "Helping self and others",
"scored": false,
"options": ["I learned more about my health and condition", "I had a positive experience with the study staff", "The research topic was of interest to me", "In order to receive compensation", "To receive additional medical attention", "Access to the newest treatment for my condition", "Other"]
}]
}
}
}
Get Responses
| Syntax | /study/identifier/survey/identifier/response
/study/identifier/response
/response
|
| Returns | Array |
| Description | Returns a list of survey Response objects, associated with either a survey, a study, or all response data |
Response Object Format
| Field |
Type |
Description |
| responseKey |
String |
Internal unique response identifier, auto-generated |
| studyKey |
String |
Internal study identifier linked to the response |
| surveyKey |
String |
Internal survey identifier linked to the response |
| siteKey |
String |
If available, the internal identifier for the response site |
| siteName |
String |
If available, the description name for the response site |
| partial |
Number |
0 (false) if the response is partial or incomplete, otherwise 1 |
| status |
String |
Complete, Partial or Disqualified; Partial and Disqualified are reserved
for future use. |
| ageGroup |
String |
Descriptive label for the respondent's age group. See the Survey Config Detail format for the list
of available age groups |
| gender |
String |
Male or Female |
| race |
String |
Descriptive label for the respondent's race or ethnicity. See the Survey Config Detail format for the list
of available ethnic categories |
| countryCode |
String |
Two-letter country code associated with the response |
| duration |
Number |
Response time in minutes |
| creationdate |
Date |
Date and time the response was added to the system (YYYY-MM-DD HH:MM:SS) |
| submissionDate |
Date |
Date and time the response was submitted by the respondent (YYYY-MM-DD HH:MM:SS) |
| answers |
Array |
List of all answers provided |
answer
questionKey |
String |
Question key for this answer. |
answer
question |
String |
Text of the question as presented to the respondent |
answer
scored |
Boolean |
true if the question was used in calculation of response metrics, otherwise false |
answer
selections |
Array |
Selected response(s) for this question |
Payload Example
{
"result_ok": true,
"utc": 1526389904,
"api_client": "CorEvitas",
"request": "response",
"return_type": "array",
"page": 1,
"total_pages": 2,
"total_items": 12,
"api_token": "772fd14c884df61f73ecd427268fce49",
"nonce": "cbbb6bd179feeee1",
"verifier": "610fea5460a5c94764e72473c178de2b200ab50d6925c8d9c401fb6357147434",
"data": [{
"responseKey": "201710-59da722fe647e:000002",
"studyKey": "201710-59da721e7b2b8",
"surveyKey": "201710-59da722fe647e",
"siteKey": null,
"siteName": null,
"partial": "0",
"status": "Complete",
"ageGroup": "30 - 39",
"gender": "Female",
"race": null,
"countryCode": "US",
"duration": "2.2",
"creationdate": "2017-10-08 14:53:29",
"submissionDate": "2017-10-08 17:47:39",
"answers": [{
"questionKey": "HV-META1",
"question": "Response ID",
"scored": false,
"selections": ["2"]
}, {
"questionKey": "HV-META2",
"question": "Start Time",
"scored": false,
"selections": ["8 October 2017 1:45:26 PM"]
}, {
"questionKey": "HV-META3",
"question": "Finish Time",
"scored": false,
"selections": ["8 October 2017 1:47:39 PM"]
}, {
"questionKey": "HV-META4",
"question": "Status",
"scored": false,
"selections": ["Complete"]
}, {
"questionKey": "HV-META6",
"question": "Language",
"scored": false,
"selections": ["English"]
}, {
"questionKey": "HV-DEM1",
"question": "What country are you in?",
"scored": false,
"selections": ["US"]
}, {
"questionKey": "HV-SCR1",
"question": "Would you like to participate in the survey?",
"scored": false,
"selections": ["Yes"]
}, {
"questionKey": "HV-META10",
"question": "Please select your study site from the list below.",
"scored": false,
"selections": ["Advanced Study Institute"]
}, {
"questionKey": "HV-DEM2",
"question": "What is your gender?",
"scored": false,
"selections": ["Female"]
}, {
"questionKey": "HV-DEM9",
"question": "How old are you?",
"scored": false,
"selections": ["30-39"]
}, {
"questionKey": "HV-DEM7",
"question": "What motivated you to join the clinical trial? (Select all that apply)",
"scored": false,
"selections": ["Curiosity to see if it would improve my condition", "To seek a better quality of life", "Free medication and\/or treatment"]
}, {
"questionKey": "HV-COM1",
"question": "During the informed consent process, how satisfied were you with the explanation of the study?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE18",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Good lighting in waiting area and exam rooms",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE19",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Close and convenient access to restrooms",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE20",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Amenities such as WIFI, magazines, TV",
"scored": true,
"selections": ["Completely satisfied"]
}, {
"questionKey": "HV-SE21",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Cleanliness of facilities",
"scored": true,
"selections": ["Slightly satisfied"]
}, {
"questionKey": "HV-SE22",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Site accommodations (comfortable seating, private space, etc.)",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE23",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Access to drinks and snacks",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-OS1",
"question": "How satisfied were you with your overall clinical research study experience?",
"scored": true,
"selections": ["Moderately satisfied"]
}, {
"questionKey": "HV-REL1",
"question": "How satisfied were you with your health concerns being addressed by the study staff?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE7",
"question": "How often was the study staff prepared for your visits?",
"scored": true,
"selections": ["Often"]
}, {
"questionKey": "HV-COM4",
"question": "How satisfied were you in general with how study staff communicated with you?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-CON10",
"question": "How easy was it taking your study medication (e.g. swallowing, injecting, etc.)?",
"scored": true,
"selections": ["Slightly easy"]
}, {
"questionKey": "HV-COM5",
"question": "How satisfied were you with the instruction provided by the study staff for how to take and store your study medication?",
"scored": true,
"selections": ["Moderately satisfied"]
}, {
"questionKey": "HV-CON5",
"question": "How convenient was parking at the study site?",
"scored": true,
"selections": ["Slightly convenient"]
}, {
"questionKey": "HV-REL8",
"question": "How helpful would the following be to support your participation in the clinical trial? Please rank the following items in order from most to least supportive.",
"scored": false,
"selections": ["Home visits or alternate location visits", "Travel Coordination", "Vouchers\/pre-payment", "24\/7 telephone support"]
}, {
"questionKey": "HV-REL3",
"question": "How often did you feel encouragement and support from the study staff?",
"scored": true,
"selections": ["Sometimes"]
}, {
"questionKey": "HV-CON9",
"question": "How easy was it to arrange child care or spouse\/parent care while you went to your study visits?",
"scored": true,
"selections": ["Not at all easy"]
}, {
"questionKey": "HV-SE11",
"question": "Were you encouraged to bring a friend or family member to the study visits?",
"scored": true,
"selections": ["Slightly encouraged"]
}, {
"questionKey": "HV-HLP1",
"question": "Did you ever consider stopping your participation in the clinical trial?",
"scored": false,
"selections": ["Yes"]
}, {
"questionKey": "HV-HLP6",
"question": "Which of the following, if any, were reasons why you considered stopping your participation in the clinical trial? (Select all that apply)",
"scored": false,
"selections": ["Family or caregiver asked me to stop", "I did not have child care"]
}, {
"questionKey": "HV-HLP3",
"question": "How likely are you to participate in another clinical trial?",
"scored": true,
"selections": ["Moderately likely"]
}, {
"questionKey": "HV-HLP4",
"question": "Why would you participate in another clinical trial? (Select all that apply)",
"scored": false,
"selections": ["To receive additional medical attention"]
}]
}, {
"responseKey": "201710-59da722fe647e:000003",
"studyKey": "201710-59da721e7b2b8",
"surveyKey": "201710-59da722fe647e",
"siteKey": null,
"siteName": null,
"partial": "0",
"status": "Complete",
"ageGroup": "50 - 59",
"gender": "Female",
"race": null,
"countryCode": "US",
"duration": "1.6",
"creationdate": "2017-10-08 14:53:29",
"submissionDate": "2017-10-08 17:49:21",
"answers": [{
"questionKey": "HV-META1",
"question": "Response ID",
"scored": false,
"selections": ["3"]
}, {
"questionKey": "HV-META2",
"question": "Start Time",
"scored": false,
"selections": ["8 October 2017 1:47:43 PM"]
}, {
"questionKey": "HV-META3",
"question": "Finish Time",
"scored": false,
"selections": ["8 October 2017 1:49:21 PM"]
}, {
"questionKey": "HV-META4",
"question": "Status",
"scored": false,
"selections": ["Complete"]
}, {
"questionKey": "HV-META6",
"question": "Language",
"scored": false,
"selections": ["English"]
}, {
"questionKey": "HV-DEM1",
"question": "What country are you in?",
"scored": false,
"selections": ["US"]
}, {
"questionKey": "HV-SCR1",
"question": "Would you like to participate in the survey?",
"scored": false,
"selections": ["Yes"]
}, {
"questionKey": "HV-META10",
"question": "Please select your study site from the list below.",
"scored": false,
"selections": ["Clinical Research Center"]
}, {
"questionKey": "HV-DEM2",
"question": "What is your gender?",
"scored": false,
"selections": ["Female"]
}, {
"questionKey": "HV-DEM9",
"question": "How old are you?",
"scored": false,
"selections": ["50-59"]
}, {
"questionKey": "HV-DEM7",
"question": "What motivated you to join the clinical trial? (Select all that apply)",
"scored": false,
"selections": ["No other treatment options were available", "Free medication and\/or treatment", "Free procedures"]
}, {
"questionKey": "HV-COM1",
"question": "During the informed consent process, how satisfied were you with the explanation of the study?",
"scored": true,
"selections": ["Moderately satisfied"]
}, {
"questionKey": "HV-SE18",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Good lighting in waiting area and exam rooms",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE19",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Close and convenient access to restrooms",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE20",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Amenities such as WIFI, magazines, TV",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE21",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Cleanliness of facilities",
"scored": true,
"selections": ["Moderately satisfied"]
}, {
"questionKey": "HV-SE22",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Site accommodations (comfortable seating, private space, etc.)",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE23",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Access to drinks and snacks",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-OS1",
"question": "How satisfied were you with your overall clinical research study experience?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-REL1",
"question": "How satisfied were you with your health concerns being addressed by the study staff?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE7",
"question": "How often was the study staff prepared for your visits?",
"scored": true,
"selections": ["Often"]
}, {
"questionKey": "HV-COM4",
"question": "How satisfied were you in general with how study staff communicated with you?",
"scored": true,
"selections": ["Completely satisfied"]
}, {
"questionKey": "HV-CON10",
"question": "How easy was it taking your study medication (e.g. swallowing, injecting, etc.)?",
"scored": true,
"selections": ["Moderately easy"]
}, {
"questionKey": "HV-COM5",
"question": "How satisfied were you with the instruction provided by the study staff for how to take and store your study medication?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-CON5",
"question": "How convenient was parking at the study site?",
"scored": true,
"selections": ["Moderately convenient"]
}, {
"questionKey": "HV-REL8",
"question": "How helpful would the following be to support your participation in the clinical trial? Please rank the following items in order from most to least supportive.",
"scored": false,
"selections": ["Home visits or alternate location visits", "24\/7 telephone support", "Travel Coordination", "Vouchers\/pre-payment"]
}, {
"questionKey": "HV-REL3",
"question": "How often did you feel encouragement and support from the study staff?",
"scored": true,
"selections": ["Rarely"]
}, {
"questionKey": "HV-CON9",
"question": "How easy was it to arrange child care or spouse\/parent care while you went to your study visits?",
"scored": true,
"selections": ["Not applicable"]
}, {
"questionKey": "HV-SE11",
"question": "Were you encouraged to bring a friend or family member to the study visits?",
"scored": true,
"selections": ["Slightly encouraged"]
}, {
"questionKey": "HV-HLP1",
"question": "Did you ever consider stopping your participation in the clinical trial?",
"scored": false,
"selections": ["No"]
}, {
"questionKey": "HV-HLP3",
"question": "How likely are you to participate in another clinical trial?",
"scored": true,
"selections": ["Very likely"]
}, {
"questionKey": "HV-HLP4",
"question": "Why would you participate in another clinical trial? (Select all that apply)",
"scored": false,
"selections": ["I had a positive experience with the study staff", "The research topic was of interest to me", "To receive additional medical attention", "Access to the newest treatment for my condition"]
}]
}, {
"responseKey": "201710-59da722fe647e:000004",
"studyKey": "201710-59da721e7b2b8",
"surveyKey": "201710-59da722fe647e",
"siteKey": null,
"siteName": null,
"partial": "0",
"status": "Complete",
"ageGroup": "50 - 59",
"gender": "Male",
"race": null,
"countryCode": "US",
"duration": "2.5",
"creationdate": "2017-10-08 14:53:29",
"submissionDate": "2017-10-08 17:51:55",
"answers": [{
"questionKey": "HV-META1",
"question": "Response ID",
"scored": false,
"selections": ["4"]
}, {
"questionKey": "HV-META2",
"question": "Start Time",
"scored": false,
"selections": ["8 October 2017 1:49:24 PM"]
}, {
"questionKey": "HV-META3",
"question": "Finish Time",
"scored": false,
"selections": ["8 October 2017 1:51:55 PM"]
}, {
"questionKey": "HV-META4",
"question": "Status",
"scored": false,
"selections": ["Complete"]
}, {
"questionKey": "HV-META6",
"question": "Language",
"scored": false,
"selections": ["English"]
}, {
"questionKey": "HV-DEM1",
"question": "What country are you in?",
"scored": false,
"selections": ["US"]
}, {
"questionKey": "HV-SCR1",
"question": "Would you like to participate in the survey?",
"scored": false,
"selections": ["Yes"]
}, {
"questionKey": "HV-META10",
"question": "Please select your study site from the list below.",
"scored": false,
"selections": ["Midwest Research Associates"]
}, {
"questionKey": "HV-DEM2",
"question": "What is your gender?",
"scored": false,
"selections": ["Male"]
}, {
"questionKey": "HV-DEM9",
"question": "How old are you?",
"scored": false,
"selections": ["50-59"]
}, {
"questionKey": "HV-DEM7",
"question": "What motivated you to join the clinical trial? (Select all that apply)",
"scored": false,
"selections": ["Curiosity to see if it would improve my condition", "To seek a better quality of life", "Free medication and\/or treatment", "Free procedures"]
}, {
"questionKey": "HV-COM1",
"question": "During the informed consent process, how satisfied were you with the explanation of the study?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE18",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Good lighting in waiting area and exam rooms",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE19",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Close and convenient access to restrooms",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE20",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Amenities such as WIFI, magazines, TV",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE21",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Cleanliness of facilities",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE22",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Site accommodations (comfortable seating, private space, etc.)",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE23",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Access to drinks and snacks",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-OS1",
"question": "How satisfied were you with your overall clinical research study experience?",
"scored": true,
"selections": ["Slightly satisfied"]
}, {
"questionKey": "HV-REL1",
"question": "How satisfied were you with your health concerns being addressed by the study staff?",
"scored": true,
"selections": ["Moderately satisfied"]
}, {
"questionKey": "HV-SE7",
"question": "How often was the study staff prepared for your visits?",
"scored": true,
"selections": ["Rarely"]
}, {
"questionKey": "HV-COM4",
"question": "How satisfied were you in general with how study staff communicated with you?",
"scored": true,
"selections": ["Slightly satisfied"]
}, {
"questionKey": "HV-CON10",
"question": "How easy was it taking your study medication (e.g. swallowing, injecting, etc.)?",
"scored": true,
"selections": ["Not at all easy"]
}, {
"questionKey": "HV-COM5",
"question": "How satisfied were you with the instruction provided by the study staff for how to take and store your study medication?",
"scored": true,
"selections": ["Slightly satisfied"]
}, {
"questionKey": "HV-CON5",
"question": "How convenient was parking at the study site?",
"scored": true,
"selections": ["Not applicable"]
}, {
"questionKey": "HV-REL8",
"question": "How helpful would the following be to support your participation in the clinical trial? Please rank the following items in order from most to least supportive.",
"scored": false,
"selections": ["24\/7 telephone support", "Vouchers\/pre-payment", "Home visits or alternate location visits", "Travel Coordination"]
}, {
"questionKey": "HV-REL3",
"question": "How often did you feel encouragement and support from the study staff?",
"scored": true,
"selections": ["Rarely"]
}, {
"questionKey": "HV-CON9",
"question": "How easy was it to arrange child care or spouse\/parent care while you went to your study visits?",
"scored": true,
"selections": ["Not applicable"]
}, {
"questionKey": "HV-SE11",
"question": "Were you encouraged to bring a friend or family member to the study visits?",
"scored": true,
"selections": ["Not at all encouraged"]
}, {
"questionKey": "HV-HLP1",
"question": "Did you ever consider stopping your participation in the clinical trial?",
"scored": false,
"selections": ["Yes"]
}, {
"questionKey": "HV-HLP6",
"question": "Which of the following, if any, were reasons why you considered stopping your participation in the clinical trial? (Select all that apply)",
"scored": false,
"selections": ["Too many study visits were required", "I thought that I was on a placebo (sugar pill, inactive pill)", "Instructions were too difficult to follow", "Worried about side effects"]
}, {
"questionKey": "HV-HLP3",
"question": "How likely are you to participate in another clinical trial?",
"scored": true,
"selections": ["Slightly likely"]
}, {
"questionKey": "HV-HLP4",
"question": "Why would you participate in another clinical trial? (Select all that apply)",
"scored": false,
"selections": ["Access to the newest treatment for my condition"]
}]
}, {
"responseKey": "201710-59da722fe647e:000005",
"studyKey": "201710-59da721e7b2b8",
"surveyKey": "201710-59da722fe647e",
"siteKey": null,
"siteName": null,
"partial": "0",
"status": "Complete",
"ageGroup": "60 - 69",
"gender": "Female",
"race": null,
"countryCode": "US",
"duration": "2",
"creationdate": "2017-10-08 14:53:29",
"submissionDate": "2017-10-08 17:54:01",
"answers": [{
"questionKey": "HV-META1",
"question": "Response ID",
"scored": false,
"selections": ["5"]
}, {
"questionKey": "HV-META2",
"question": "Start Time",
"scored": false,
"selections": ["8 October 2017 1:51:58 PM"]
}, {
"questionKey": "HV-META3",
"question": "Finish Time",
"scored": false,
"selections": ["8 October 2017 1:54:01 PM"]
}, {
"questionKey": "HV-META4",
"question": "Status",
"scored": false,
"selections": ["Complete"]
}, {
"questionKey": "HV-META6",
"question": "Language",
"scored": false,
"selections": ["English"]
}, {
"questionKey": "HV-DEM1",
"question": "What country are you in?",
"scored": false,
"selections": ["US"]
}, {
"questionKey": "HV-SCR1",
"question": "Would you like to participate in the survey?",
"scored": false,
"selections": ["Yes"]
}, {
"questionKey": "HV-META10",
"question": "Please select your study site from the list below.",
"scored": false,
"selections": ["Advanced Study Institute"]
}, {
"questionKey": "HV-DEM2",
"question": "What is your gender?",
"scored": false,
"selections": ["Female"]
}, {
"questionKey": "HV-DEM9",
"question": "How old are you?",
"scored": false,
"selections": ["60-69"]
}, {
"questionKey": "HV-DEM7",
"question": "What motivated you to join the clinical trial? (Select all that apply)",
"scored": false,
"selections": ["Curiosity to see if it would improve my condition", "Contribute to research and help people live longer"]
}, {
"questionKey": "HV-COM1",
"question": "During the informed consent process, how satisfied were you with the explanation of the study?",
"scored": true,
"selections": ["Slightly satisfied"]
}, {
"questionKey": "HV-SE18",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Good lighting in waiting area and exam rooms",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE19",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Close and convenient access to restrooms",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE20",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Amenities such as WIFI, magazines, TV",
"scored": true,
"selections": ["Moderately satisfied"]
}, {
"questionKey": "HV-SE21",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Cleanliness of facilities",
"scored": true,
"selections": ["Moderately satisfied"]
}, {
"questionKey": "HV-SE22",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Site accommodations (comfortable seating, private space, etc.)",
"scored": true,
"selections": ["Moderately satisfied"]
}, {
"questionKey": "HV-SE23",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Access to drinks and snacks",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-OS1",
"question": "How satisfied were you with your overall clinical research study experience?",
"scored": true,
"selections": ["Slightly satisfied"]
}, {
"questionKey": "HV-REL1",
"question": "How satisfied were you with your health concerns being addressed by the study staff?",
"scored": true,
"selections": ["Slightly satisfied"]
}, {
"questionKey": "HV-SE7",
"question": "How often was the study staff prepared for your visits?",
"scored": true,
"selections": ["Rarely"]
}, {
"questionKey": "HV-COM4",
"question": "How satisfied were you in general with how study staff communicated with you?",
"scored": true,
"selections": ["Slightly satisfied"]
}, {
"questionKey": "HV-CON10",
"question": "How easy was it taking your study medication (e.g. swallowing, injecting, etc.)?",
"scored": true,
"selections": ["Moderately easy"]
}, {
"questionKey": "HV-COM5",
"question": "How satisfied were you with the instruction provided by the study staff for how to take and store your study medication?",
"scored": true,
"selections": ["Moderately satisfied"]
}, {
"questionKey": "HV-CON5",
"question": "How convenient was parking at the study site?",
"scored": true,
"selections": ["Slightly convenient"]
}, {
"questionKey": "HV-REL8",
"question": "How helpful would the following be to support your participation in the clinical trial? Please rank the following items in order from most to least supportive.",
"scored": false,
"selections": ["Home visits or alternate location visits", "Vouchers\/pre-payment", "Travel Coordination", "24\/7 telephone support"]
}, {
"questionKey": "HV-REL3",
"question": "How often did you feel encouragement and support from the study staff?",
"scored": true,
"selections": ["Sometimes"]
}, {
"questionKey": "HV-CON9",
"question": "How easy was it to arrange child care or spouse\/parent care while you went to your study visits?",
"scored": true,
"selections": ["Slightly easy"]
}, {
"questionKey": "HV-SE11",
"question": "Were you encouraged to bring a friend or family member to the study visits?",
"scored": true,
"selections": ["Slightly encouraged"]
}, {
"questionKey": "HV-HLP1",
"question": "Did you ever consider stopping your participation in the clinical trial?",
"scored": false,
"selections": ["Yes"]
}, {
"questionKey": "HV-HLP6",
"question": "Which of the following, if any, were reasons why you considered stopping your participation in the clinical trial? (Select all that apply)",
"scored": false,
"selections": ["The study staff were not friendly", "I did not feel appreciated", "I felt I was not being informed about my health"]
}, {
"questionKey": "HV-HLP3",
"question": "How likely are you to participate in another clinical trial?",
"scored": true,
"selections": ["Slightly likely"]
}, {
"questionKey": "HV-HLP4",
"question": "Why would you participate in another clinical trial? (Select all that apply)",
"scored": false,
"selections": ["To receive additional medical attention"]
}]
}, {
"responseKey": "201710-59da722fe647e:000006",
"studyKey": "201710-59da721e7b2b8",
"surveyKey": "201710-59da722fe647e",
"siteKey": null,
"siteName": null,
"partial": "0",
"status": "Complete",
"ageGroup": "50 - 59",
"gender": "Male",
"race": null,
"countryCode": "US",
"duration": "1.8",
"creationdate": "2017-10-08 14:53:29",
"submissionDate": "2017-10-08 17:55:55",
"answers": [{
"questionKey": "HV-META1",
"question": "Response ID",
"scored": false,
"selections": ["6"]
}, {
"questionKey": "HV-META2",
"question": "Start Time",
"scored": false,
"selections": ["8 October 2017 1:54:05 PM"]
}, {
"questionKey": "HV-META3",
"question": "Finish Time",
"scored": false,
"selections": ["8 October 2017 1:55:55 PM"]
}, {
"questionKey": "HV-META4",
"question": "Status",
"scored": false,
"selections": ["Complete"]
}, {
"questionKey": "HV-META6",
"question": "Language",
"scored": false,
"selections": ["English"]
}, {
"questionKey": "HV-DEM1",
"question": "What country are you in?",
"scored": false,
"selections": ["US"]
}, {
"questionKey": "HV-SCR1",
"question": "Would you like to participate in the survey?",
"scored": false,
"selections": ["Yes"]
}, {
"questionKey": "HV-META10",
"question": "Please select your study site from the list below.",
"scored": false,
"selections": ["Clinical Research Center"]
}, {
"questionKey": "HV-DEM2",
"question": "What is your gender?",
"scored": false,
"selections": ["Male"]
}, {
"questionKey": "HV-DEM9",
"question": "How old are you?",
"scored": false,
"selections": ["50-59"]
}, {
"questionKey": "HV-DEM7",
"question": "What motivated you to join the clinical trial? (Select all that apply)",
"scored": false,
"selections": ["Curiosity to see if it would improve my condition", "Contribute to research and help people live longer", "To seek a better quality of life", "No other treatment options were available"]
}, {
"questionKey": "HV-COM1",
"question": "During the informed consent process, how satisfied were you with the explanation of the study?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE18",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Good lighting in waiting area and exam rooms",
"scored": true,
"selections": ["Completely satisfied"]
}, {
"questionKey": "HV-SE19",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Close and convenient access to restrooms",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE20",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Amenities such as WIFI, magazines, TV",
"scored": true,
"selections": ["Completely satisfied"]
}, {
"questionKey": "HV-SE21",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Cleanliness of facilities",
"scored": true,
"selections": ["Completely satisfied"]
}, {
"questionKey": "HV-SE22",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Site accommodations (comfortable seating, private space, etc.)",
"scored": true,
"selections": ["Completely satisfied"]
}, {
"questionKey": "HV-SE23",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Access to drinks and snacks",
"scored": true,
"selections": ["Completely satisfied"]
}, {
"questionKey": "HV-OS1",
"question": "How satisfied were you with your overall clinical research study experience?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-REL1",
"question": "How satisfied were you with your health concerns being addressed by the study staff?",
"scored": true,
"selections": ["Completely satisfied"]
}, {
"questionKey": "HV-SE7",
"question": "How often was the study staff prepared for your visits?",
"scored": true,
"selections": ["Often"]
}, {
"questionKey": "HV-COM4",
"question": "How satisfied were you in general with how study staff communicated with you?",
"scored": true,
"selections": ["Completely satisfied"]
}, {
"questionKey": "HV-CON10",
"question": "How easy was it taking your study medication (e.g. swallowing, injecting, etc.)?",
"scored": true,
"selections": ["Moderately easy"]
}, {
"questionKey": "HV-COM5",
"question": "How satisfied were you with the instruction provided by the study staff for how to take and store your study medication?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-CON5",
"question": "How convenient was parking at the study site?",
"scored": true,
"selections": ["Moderately convenient"]
}, {
"questionKey": "HV-REL8",
"question": "How helpful would the following be to support your participation in the clinical trial? Please rank the following items in order from most to least supportive.",
"scored": false,
"selections": ["24\/7 telephone support", "Vouchers\/pre-payment", "Home visits or alternate location visits", "Travel Coordination"]
}, {
"questionKey": "HV-REL3",
"question": "How often did you feel encouragement and support from the study staff?",
"scored": true,
"selections": ["Sometimes"]
}, {
"questionKey": "HV-CON9",
"question": "How easy was it to arrange child care or spouse\/parent care while you went to your study visits?",
"scored": true,
"selections": ["Not applicable"]
}, {
"questionKey": "HV-SE11",
"question": "Were you encouraged to bring a friend or family member to the study visits?",
"scored": true,
"selections": ["Moderately encouraged"]
}, {
"questionKey": "HV-HLP1",
"question": "Did you ever consider stopping your participation in the clinical trial?",
"scored": false,
"selections": ["No"]
}, {
"questionKey": "HV-HLP3",
"question": "How likely are you to participate in another clinical trial?",
"scored": true,
"selections": ["Very likely"]
}, {
"questionKey": "HV-HLP4",
"question": "Why would you participate in another clinical trial? (Select all that apply)",
"scored": false,
"selections": ["I learned more about my health and condition", "I had a positive experience with the study staff", "To receive additional medical attention", "Access to the newest treatment for my condition"]
}]
}, {
"responseKey": "201710-59da722fe647e:000007",
"studyKey": "201710-59da721e7b2b8",
"surveyKey": "201710-59da722fe647e",
"siteKey": null,
"siteName": null,
"partial": "0",
"status": "Complete",
"ageGroup": "60 - 69",
"gender": "Female",
"race": null,
"countryCode": "US",
"duration": "2.1",
"creationdate": "2017-10-08 14:53:29",
"submissionDate": "2017-10-08 17:58:09",
"answers": [{
"questionKey": "HV-META1",
"question": "Response ID",
"scored": false,
"selections": ["7"]
}, {
"questionKey": "HV-META2",
"question": "Start Time",
"scored": false,
"selections": ["8 October 2017 1:55:58 PM"]
}, {
"questionKey": "HV-META3",
"question": "Finish Time",
"scored": false,
"selections": ["8 October 2017 1:58:09 PM"]
}, {
"questionKey": "HV-META4",
"question": "Status",
"scored": false,
"selections": ["Complete"]
}, {
"questionKey": "HV-META6",
"question": "Language",
"scored": false,
"selections": ["English"]
}, {
"questionKey": "HV-DEM1",
"question": "What country are you in?",
"scored": false,
"selections": ["US"]
}, {
"questionKey": "HV-SCR1",
"question": "Would you like to participate in the survey?",
"scored": false,
"selections": ["Yes"]
}, {
"questionKey": "HV-META10",
"question": "Please select your study site from the list below.",
"scored": false,
"selections": ["West Research Center"]
}, {
"questionKey": "HV-DEM2",
"question": "What is your gender?",
"scored": false,
"selections": ["Female"]
}, {
"questionKey": "HV-DEM9",
"question": "How old are you?",
"scored": false,
"selections": ["60-69"]
}, {
"questionKey": "HV-DEM7",
"question": "What motivated you to join the clinical trial? (Select all that apply)",
"scored": false,
"selections": ["Curiosity to see if it would improve my condition", "To seek a better quality of life", "No other treatment options were available", "Free medication and\/or treatment"]
}, {
"questionKey": "HV-COM1",
"question": "During the informed consent process, how satisfied were you with the explanation of the study?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE18",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Good lighting in waiting area and exam rooms",
"scored": true,
"selections": ["Completely satisfied"]
}, {
"questionKey": "HV-SE19",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Close and convenient access to restrooms",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE20",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Amenities such as WIFI, magazines, TV",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE21",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Cleanliness of facilities",
"scored": true,
"selections": ["Completely satisfied"]
}, {
"questionKey": "HV-SE22",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Site accommodations (comfortable seating, private space, etc.)",
"scored": true,
"selections": ["Completely satisfied"]
}, {
"questionKey": "HV-SE23",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Access to drinks and snacks",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-OS1",
"question": "How satisfied were you with your overall clinical research study experience?",
"scored": true,
"selections": ["Moderately satisfied"]
}, {
"questionKey": "HV-REL1",
"question": "How satisfied were you with your health concerns being addressed by the study staff?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE7",
"question": "How often was the study staff prepared for your visits?",
"scored": true,
"selections": ["Often"]
}, {
"questionKey": "HV-COM4",
"question": "How satisfied were you in general with how study staff communicated with you?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-CON10",
"question": "How easy was it taking your study medication (e.g. swallowing, injecting, etc.)?",
"scored": true,
"selections": ["Moderately easy"]
}, {
"questionKey": "HV-COM5",
"question": "How satisfied were you with the instruction provided by the study staff for how to take and store your study medication?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-CON5",
"question": "How convenient was parking at the study site?",
"scored": true,
"selections": ["Not at all convenient"]
}, {
"questionKey": "HV-REL8",
"question": "How helpful would the following be to support your participation in the clinical trial? Please rank the following items in order from most to least supportive.",
"scored": false,
"selections": ["Home visits or alternate location visits", "Vouchers\/pre-payment", "Travel Coordination", "24\/7 telephone support"]
}, {
"questionKey": "HV-REL3",
"question": "How often did you feel encouragement and support from the study staff?",
"scored": true,
"selections": ["Rarely"]
}, {
"questionKey": "HV-CON9",
"question": "How easy was it to arrange child care or spouse\/parent care while you went to your study visits?",
"scored": true,
"selections": ["Not at all easy"]
}, {
"questionKey": "HV-SE11",
"question": "Were you encouraged to bring a friend or family member to the study visits?",
"scored": true,
"selections": ["Slightly encouraged"]
}, {
"questionKey": "HV-HLP1",
"question": "Did you ever consider stopping your participation in the clinical trial?",
"scored": false,
"selections": ["No"]
}, {
"questionKey": "HV-HLP3",
"question": "How likely are you to participate in another clinical trial?",
"scored": true,
"selections": ["Slightly likely"]
}, {
"questionKey": "HV-HLP4",
"question": "Why would you participate in another clinical trial? (Select all that apply)",
"scored": false,
"selections": ["I learned more about my health and condition", "To receive additional medical attention", "Access to the newest treatment for my condition"]
}]
}, {
"responseKey": "201710-59da722fe647e:000008",
"studyKey": "201710-59da721e7b2b8",
"surveyKey": "201710-59da722fe647e",
"siteKey": null,
"siteName": null,
"partial": "0",
"status": "Complete",
"ageGroup": "30 - 39",
"gender": "Female",
"race": null,
"countryCode": "CA",
"duration": "1.5",
"creationdate": "2017-10-08 14:53:29",
"submissionDate": "2017-10-08 18:00:33",
"answers": [{
"questionKey": "HV-META1",
"question": "Response ID",
"scored": false,
"selections": ["8"]
}, {
"questionKey": "HV-META2",
"question": "Start Time",
"scored": false,
"selections": ["8 October 2017 1:59:03 PM"]
}, {
"questionKey": "HV-META3",
"question": "Finish Time",
"scored": false,
"selections": ["8 October 2017 2:00:33 PM"]
}, {
"questionKey": "HV-META4",
"question": "Status",
"scored": false,
"selections": ["Complete"]
}, {
"questionKey": "HV-META6",
"question": "Language",
"scored": false,
"selections": ["English"]
}, {
"questionKey": "HV-DEM1",
"question": "What country are you in?",
"scored": false,
"selections": ["CA"]
}, {
"questionKey": "HV-SCR1",
"question": "Would you like to participate in the survey?",
"scored": false,
"selections": ["Yes"]
}, {
"questionKey": "HV-META10",
"question": "Please select your study site from the list below.",
"scored": false,
"selections": ["Montreal Clinical Trial Institute"]
}, {
"questionKey": "HV-DEM2",
"question": "What is your gender?",
"scored": false,
"selections": ["Female"]
}, {
"questionKey": "HV-DEM9",
"question": "How old are you?",
"scored": false,
"selections": ["30-39"]
}, {
"questionKey": "HV-DEM7",
"question": "What motivated you to join the clinical trial? (Select all that apply)",
"scored": false,
"selections": ["Curiosity to see if it would improve my condition", "Contribute to research and help people live longer", "To seek a better quality of life"]
}, {
"questionKey": "HV-COM1",
"question": "During the informed consent process, how satisfied were you with the explanation of the study?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE18",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Good lighting in waiting area and exam rooms",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE19",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Close and convenient access to restrooms",
"scored": true,
"selections": ["Completely satisfied"]
}, {
"questionKey": "HV-SE20",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Amenities such as WIFI, magazines, TV",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE21",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Cleanliness of facilities",
"scored": true,
"selections": ["Completely satisfied"]
}, {
"questionKey": "HV-SE22",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Site accommodations (comfortable seating, private space, etc.)",
"scored": true,
"selections": ["Completely satisfied"]
}, {
"questionKey": "HV-SE23",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Access to drinks and snacks",
"scored": true,
"selections": ["Completely satisfied"]
}, {
"questionKey": "HV-OS1",
"question": "How satisfied were you with your overall clinical research study experience?",
"scored": true,
"selections": ["Completely satisfied"]
}, {
"questionKey": "HV-REL1",
"question": "How satisfied were you with your health concerns being addressed by the study staff?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE7",
"question": "How often was the study staff prepared for your visits?",
"scored": true,
"selections": ["Always"]
}, {
"questionKey": "HV-COM4",
"question": "How satisfied were you in general with how study staff communicated with you?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-CON10",
"question": "How easy was it taking your study medication (e.g. swallowing, injecting, etc.)?",
"scored": true,
"selections": ["Extremely easy"]
}, {
"questionKey": "HV-COM5",
"question": "How satisfied were you with the instruction provided by the study staff for how to take and store your study medication?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-CON5",
"question": "How convenient was parking at the study site?",
"scored": true,
"selections": ["Not applicable"]
}, {
"questionKey": "HV-REL8",
"question": "How helpful would the following be to support your participation in the clinical trial? Please rank the following items in order from most to least supportive.",
"scored": false,
"selections": ["24\/7 telephone support", "Travel Coordination", "Vouchers\/pre-payment", "Home visits or alternate location visits"]
}, {
"questionKey": "HV-REL3",
"question": "How often did you feel encouragement and support from the study staff?",
"scored": true,
"selections": ["Often"]
}, {
"questionKey": "HV-CON9",
"question": "How easy was it to arrange child care or spouse\/parent care while you went to your study visits?",
"scored": true,
"selections": ["Extremely easy"]
}, {
"questionKey": "HV-SE11",
"question": "Were you encouraged to bring a friend or family member to the study visits?",
"scored": true,
"selections": ["Very encouraged"]
}, {
"questionKey": "HV-HLP1",
"question": "Did you ever consider stopping your participation in the clinical trial?",
"scored": false,
"selections": ["No"]
}, {
"questionKey": "HV-HLP3",
"question": "How likely are you to participate in another clinical trial?",
"scored": true,
"selections": ["Extremely likely"]
}, {
"questionKey": "HV-HLP4",
"question": "Why would you participate in another clinical trial? (Select all that apply)",
"scored": false,
"selections": ["In order to receive compensation", "To receive additional medical attention", "Access to the newest treatment for my condition"]
}]
}, {
"responseKey": "201710-59da722fe647e:000009",
"studyKey": "201710-59da721e7b2b8",
"surveyKey": "201710-59da722fe647e",
"siteKey": null,
"siteName": null,
"partial": "0",
"status": "Complete",
"ageGroup": "50 - 59",
"gender": "Female",
"race": null,
"countryCode": "CA",
"duration": "1.7",
"creationdate": "2017-10-08 14:53:30",
"submissionDate": "2017-10-08 18:02:19",
"answers": [{
"questionKey": "HV-META1",
"question": "Response ID",
"scored": false,
"selections": ["9"]
}, {
"questionKey": "HV-META2",
"question": "Start Time",
"scored": false,
"selections": ["8 October 2017 2:00:36 PM"]
}, {
"questionKey": "HV-META3",
"question": "Finish Time",
"scored": false,
"selections": ["8 October 2017 2:02:19 PM"]
}, {
"questionKey": "HV-META4",
"question": "Status",
"scored": false,
"selections": ["Complete"]
}, {
"questionKey": "HV-META6",
"question": "Language",
"scored": false,
"selections": ["English"]
}, {
"questionKey": "HV-DEM1",
"question": "What country are you in?",
"scored": false,
"selections": ["CA"]
}, {
"questionKey": "HV-SCR1",
"question": "Would you like to participate in the survey?",
"scored": false,
"selections": ["Yes"]
}, {
"questionKey": "HV-META10",
"question": "Please select your study site from the list below.",
"scored": false,
"selections": ["Toronto Research Center"]
}, {
"questionKey": "HV-DEM2",
"question": "What is your gender?",
"scored": false,
"selections": ["Female"]
}, {
"questionKey": "HV-DEM9",
"question": "How old are you?",
"scored": false,
"selections": ["50-59"]
}, {
"questionKey": "HV-DEM7",
"question": "What motivated you to join the clinical trial? (Select all that apply)",
"scored": false,
"selections": ["Curiosity to see if it would improve my condition", "Contribute to research and help people live longer", "To seek a better quality of life", "Free medication and\/or treatment"]
}, {
"questionKey": "HV-COM1",
"question": "During the informed consent process, how satisfied were you with the explanation of the study?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE18",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Good lighting in waiting area and exam rooms",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE19",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Close and convenient access to restrooms",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE20",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Amenities such as WIFI, magazines, TV",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE21",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Cleanliness of facilities",
"scored": true,
"selections": ["Slightly satisfied"]
}, {
"questionKey": "HV-SE22",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Site accommodations (comfortable seating, private space, etc.)",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE23",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Access to drinks and snacks",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-OS1",
"question": "How satisfied were you with your overall clinical research study experience?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-REL1",
"question": "How satisfied were you with your health concerns being addressed by the study staff?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE7",
"question": "How often was the study staff prepared for your visits?",
"scored": true,
"selections": ["Often"]
}, {
"questionKey": "HV-COM4",
"question": "How satisfied were you in general with how study staff communicated with you?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-CON10",
"question": "How easy was it taking your study medication (e.g. swallowing, injecting, etc.)?",
"scored": true,
"selections": ["Very easy"]
}, {
"questionKey": "HV-COM5",
"question": "How satisfied were you with the instruction provided by the study staff for how to take and store your study medication?",
"scored": true,
"selections": ["Completely satisfied"]
}, {
"questionKey": "HV-CON5",
"question": "How convenient was parking at the study site?",
"scored": true,
"selections": ["Extremely convenient"]
}, {
"questionKey": "HV-REL8",
"question": "How helpful would the following be to support your participation in the clinical trial? Please rank the following items in order from most to least supportive.",
"scored": false,
"selections": ["Travel Coordination", "24\/7 telephone support", "Home visits or alternate location visits", "Vouchers\/pre-payment"]
}, {
"questionKey": "HV-REL3",
"question": "How often did you feel encouragement and support from the study staff?",
"scored": true,
"selections": ["Often"]
}, {
"questionKey": "HV-CON9",
"question": "How easy was it to arrange child care or spouse\/parent care while you went to your study visits?",
"scored": true,
"selections": ["Moderately easy"]
}, {
"questionKey": "HV-SE11",
"question": "Were you encouraged to bring a friend or family member to the study visits?",
"scored": true,
"selections": ["Moderately encouraged"]
}, {
"questionKey": "HV-HLP1",
"question": "Did you ever consider stopping your participation in the clinical trial?",
"scored": false,
"selections": ["No"]
}, {
"questionKey": "HV-HLP3",
"question": "How likely are you to participate in another clinical trial?",
"scored": true,
"selections": ["Very likely"]
}, {
"questionKey": "HV-HLP4",
"question": "Why would you participate in another clinical trial? (Select all that apply)",
"scored": false,
"selections": ["I had a positive experience with the study staff", "The research topic was of interest to me", "In order to receive compensation", "Access to the newest treatment for my condition"]
}]
}, {
"responseKey": "201710-59da722fe647e:000010",
"studyKey": "201710-59da721e7b2b8",
"surveyKey": "201710-59da722fe647e",
"siteKey": null,
"siteName": null,
"partial": "0",
"status": "Complete",
"ageGroup": "40 - 49",
"gender": "Male",
"race": null,
"countryCode": "CA",
"duration": "2",
"creationdate": "2017-10-08 14:53:30",
"submissionDate": "2017-10-08 18:04:25",
"answers": [{
"questionKey": "HV-META1",
"question": "Response ID",
"scored": false,
"selections": ["10"]
}, {
"questionKey": "HV-META2",
"question": "Start Time",
"scored": false,
"selections": ["8 October 2017 2:02:22 PM"]
}, {
"questionKey": "HV-META3",
"question": "Finish Time",
"scored": false,
"selections": ["8 October 2017 2:04:25 PM"]
}, {
"questionKey": "HV-META4",
"question": "Status",
"scored": false,
"selections": ["Complete"]
}, {
"questionKey": "HV-META6",
"question": "Language",
"scored": false,
"selections": ["English"]
}, {
"questionKey": "HV-DEM1",
"question": "What country are you in?",
"scored": false,
"selections": ["CA"]
}, {
"questionKey": "HV-SCR1",
"question": "Would you like to participate in the survey?",
"scored": false,
"selections": ["Yes"]
}, {
"questionKey": "HV-META10",
"question": "Please select your study site from the list below.",
"scored": false,
"selections": ["Montreal Clinical Trial Institute"]
}, {
"questionKey": "HV-DEM2",
"question": "What is your gender?",
"scored": false,
"selections": ["Male"]
}, {
"questionKey": "HV-DEM9",
"question": "How old are you?",
"scored": false,
"selections": ["40-49"]
}, {
"questionKey": "HV-DEM7",
"question": "What motivated you to join the clinical trial? (Select all that apply)",
"scored": false,
"selections": ["Curiosity to see if it would improve my condition", "Contribute to research and help people live longer", "No other treatment options were available", "Compensation"]
}, {
"questionKey": "HV-COM1",
"question": "During the informed consent process, how satisfied were you with the explanation of the study?",
"scored": true,
"selections": ["Moderately satisfied"]
}, {
"questionKey": "HV-SE18",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Good lighting in waiting area and exam rooms",
"scored": true,
"selections": ["Moderately satisfied"]
}, {
"questionKey": "HV-SE19",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Close and convenient access to restrooms",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE20",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Amenities such as WIFI, magazines, TV",
"scored": true,
"selections": ["Moderately satisfied"]
}, {
"questionKey": "HV-SE21",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Cleanliness of facilities",
"scored": true,
"selections": ["Slightly satisfied"]
}, {
"questionKey": "HV-SE22",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Site accommodations (comfortable seating, private space, etc.)",
"scored": true,
"selections": ["Moderately satisfied"]
}, {
"questionKey": "HV-SE23",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Access to drinks and snacks",
"scored": true,
"selections": ["Moderately satisfied"]
}, {
"questionKey": "HV-OS1",
"question": "How satisfied were you with your overall clinical research study experience?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-REL1",
"question": "How satisfied were you with your health concerns being addressed by the study staff?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE7",
"question": "How often was the study staff prepared for your visits?",
"scored": true,
"selections": ["Sometimes"]
}, {
"questionKey": "HV-COM4",
"question": "How satisfied were you in general with how study staff communicated with you?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-CON10",
"question": "How easy was it taking your study medication (e.g. swallowing, injecting, etc.)?",
"scored": true,
"selections": ["Moderately easy"]
}, {
"questionKey": "HV-COM5",
"question": "How satisfied were you with the instruction provided by the study staff for how to take and store your study medication?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-CON5",
"question": "How convenient was parking at the study site?",
"scored": true,
"selections": ["Moderately convenient"]
}, {
"questionKey": "HV-REL8",
"question": "How helpful would the following be to support your participation in the clinical trial? Please rank the following items in order from most to least supportive.",
"scored": false,
"selections": ["24\/7 telephone support", "Home visits or alternate location visits", "Travel Coordination", "Vouchers\/pre-payment"]
}, {
"questionKey": "HV-REL3",
"question": "How often did you feel encouragement and support from the study staff?",
"scored": true,
"selections": ["Often"]
}, {
"questionKey": "HV-CON9",
"question": "How easy was it to arrange child care or spouse\/parent care while you went to your study visits?",
"scored": true,
"selections": ["Not applicable"]
}, {
"questionKey": "HV-SE11",
"question": "Were you encouraged to bring a friend or family member to the study visits?",
"scored": true,
"selections": ["Very encouraged"]
}, {
"questionKey": "HV-HLP1",
"question": "Did you ever consider stopping your participation in the clinical trial?",
"scored": false,
"selections": ["Yes"]
}, {
"questionKey": "HV-HLP6",
"question": "Which of the following, if any, were reasons why you considered stopping your participation in the clinical trial? (Select all that apply)",
"scored": false,
"selections": ["Too many study visits were required", "Too much time involved"]
}, {
"questionKey": "HV-HLP3",
"question": "How likely are you to participate in another clinical trial?",
"scored": true,
"selections": ["Very likely"]
}, {
"questionKey": "HV-HLP4",
"question": "Why would you participate in another clinical trial? (Select all that apply)",
"scored": false,
"selections": ["In order to receive compensation", "To receive additional medical attention", "Access to the newest treatment for my condition"]
}]
}, {
"responseKey": "201710-59da722fe647e:000011",
"studyKey": "201710-59da721e7b2b8",
"surveyKey": "201710-59da722fe647e",
"siteKey": null,
"siteName": null,
"partial": "0",
"status": "Complete",
"ageGroup": "40 - 49",
"gender": "Male",
"race": null,
"countryCode": "CA",
"duration": "1.5",
"creationdate": "2017-10-08 14:53:30",
"submissionDate": "2017-10-08 18:06:00",
"answers": [{
"questionKey": "HV-META1",
"question": "Response ID",
"scored": false,
"selections": ["11"]
}, {
"questionKey": "HV-META2",
"question": "Start Time",
"scored": false,
"selections": ["8 October 2017 2:04:29 PM"]
}, {
"questionKey": "HV-META3",
"question": "Finish Time",
"scored": false,
"selections": ["8 October 2017 2:06:00 PM"]
}, {
"questionKey": "HV-META4",
"question": "Status",
"scored": false,
"selections": ["Complete"]
}, {
"questionKey": "HV-META6",
"question": "Language",
"scored": false,
"selections": ["English"]
}, {
"questionKey": "HV-DEM1",
"question": "What country are you in?",
"scored": false,
"selections": ["CA"]
}, {
"questionKey": "HV-SCR1",
"question": "Would you like to participate in the survey?",
"scored": false,
"selections": ["Yes"]
}, {
"questionKey": "HV-META10",
"question": "Please select your study site from the list below.",
"scored": false,
"selections": ["Toronto Research Center"]
}, {
"questionKey": "HV-DEM2",
"question": "What is your gender?",
"scored": false,
"selections": ["Male"]
}, {
"questionKey": "HV-DEM9",
"question": "How old are you?",
"scored": false,
"selections": ["40-49"]
}, {
"questionKey": "HV-DEM7",
"question": "What motivated you to join the clinical trial? (Select all that apply)",
"scored": false,
"selections": ["Contribute to research and help people live longer", "To seek a better quality of life", "No other treatment options were available", "Other"]
}, {
"questionKey": "HV-COM1",
"question": "During the informed consent process, how satisfied were you with the explanation of the study?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE18",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Good lighting in waiting area and exam rooms",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE19",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Close and convenient access to restrooms",
"scored": true,
"selections": ["Completely satisfied"]
}, {
"questionKey": "HV-SE20",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Amenities such as WIFI, magazines, TV",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE21",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Cleanliness of facilities",
"scored": true,
"selections": ["Slightly satisfied"]
}, {
"questionKey": "HV-SE22",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Site accommodations (comfortable seating, private space, etc.)",
"scored": true,
"selections": ["Completely satisfied"]
}, {
"questionKey": "HV-SE23",
"question": "Please tell us how satisfied you were with the facilities at the study site.: Access to drinks and snacks",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-OS1",
"question": "How satisfied were you with your overall clinical research study experience?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-REL1",
"question": "How satisfied were you with your health concerns being addressed by the study staff?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-SE7",
"question": "How often was the study staff prepared for your visits?",
"scored": true,
"selections": ["Often"]
}, {
"questionKey": "HV-COM4",
"question": "How satisfied were you in general with how study staff communicated with you?",
"scored": true,
"selections": ["Completely satisfied"]
}, {
"questionKey": "HV-CON10",
"question": "How easy was it taking your study medication (e.g. swallowing, injecting, etc.)?",
"scored": true,
"selections": ["Moderately easy"]
}, {
"questionKey": "HV-COM5",
"question": "How satisfied were you with the instruction provided by the study staff for how to take and store your study medication?",
"scored": true,
"selections": ["Very satisfied"]
}, {
"questionKey": "HV-CON5",
"question": "How convenient was parking at the study site?",
"scored": true,
"selections": ["Moderately convenient"]
}, {
"questionKey": "HV-REL8",
"question": "How helpful would the following be to support your participation in the clinical trial? Please rank the following items in order from most to least supportive.",
"scored": false,
"selections": ["Home visits or alternate location visits", "Travel Coordination", "24\/7 telephone support", "Vouchers\/pre-payment"]
}, {
"questionKey": "HV-REL3",
"question": "How often did you feel encouragement and support from the study staff?",
"scored": true,
"selections": ["Often"]
}, {
"questionKey": "HV-CON9",
"question": "How easy was it to arrange child care or spouse\/parent care while you went to your study visits?",
"scored": true,
"selections": ["Very easy"]
}, {
"questionKey": "HV-SE11",
"question": "Were you encouraged to bring a friend or family member to the study visits?",
"scored": true,
"selections": ["Very encouraged"]
}, {
"questionKey": "HV-HLP1",
"question": "Did you ever consider stopping your participation in the clinical trial?",
"scored": false,
"selections": ["No"]
}, {
"questionKey": "HV-HLP3",
"question": "How likely are you to participate in another clinical trial?",
"scored": true,
"selections": ["Very likely"]
}, {
"questionKey": "HV-HLP4",
"question": "Why would you participate in another clinical trial? (Select all that apply)",
"scored": false,
"selections": ["I learned more about my health and condition", "I had a positive experience with the study staff", "In order to receive compensation", "Access to the newest treatment for my condition"]
}]
}]
}